Environment & Energy
Related: About this forumConfinement Effects on Moisture-Swing Direct Air Capture of Carbon Dioxide From the Atmosphere.
The paper I'll discuss in this post is this one: Confinement Effects on Moisture-Swing Direct Air Capture Yaguang Zhu, Austin Booth, and Kelsey B. Hatzell Environmental Science & Technology Letters 2024 11 (2), 89-94
Let me start here: Direct air capture, about which a great deal has been written, only makes sense in the case where fossil fuels are completely phased out, something that is only possible in my view, in a world 80 to 90% powered by nuclear energy, and zero fossil fuels, a world that doesn't exist, and will not exist at any time in the near future, as a result of the successful application of pernicious and frankly deadly, selective attention by the antinuke community, which has succeeded in leaving the planet in flames.
I don't think the word "congratulations" is in order for these types in remarking on their success at demonizing nuclear energy.
The paper is out of the Princeton University Andlinger Center, which is antinuke hell in my opinion, unless the nuclear energy in question is fusion, which like the so called "renewable energy" nirvana, hasn't come, isn't here, and most likely will never come. It is important to note that no form of alternate energy has succeeded in eliminating fossil fuels, and nuclear fission is the only proved technology than can even approach doing so, but regrettably for the future of humanity and the planet as a whole, antinukism still has a terrible hold on public consciousness.
We should never confuse popularity wisdom.
I've been around the Andlinger Center and its scientists - some of whom are surely impressive scientists - but we should disabuse ourselves of the concept that being bright, even to the extent of being described as the awful word "genius," implies wisdom. Being intelligent, even highly intelligent, is very different than being wise.
I no longer take them all that seriously for practical energy approaches. It strikes me that their all dying from the "renewable energy" Kool-Aid, which I regard as the reason the planet is dying.
Further, there are many reasons in my view why capture from air is less than ideal. The oceans extract carbon dioxide from air, and the volumetric concentration is much higher than it is in air, and thus seawater is a better tool for extracting carbon from the environment than is air.
I personally believe it is feasible to remove carbon dioxide from the environment, but as the paper points out, this requires energy. If we are using energy produced by dangerous fossil fuels for the exercise, what we have, in effect, is a perpetual motion machine of the worst kind.
From the text:
Strongly basic ion-exchange resins with quaternary ammonium (NR4+) are capable of moisture-swing direct air capture. Modifying cation, anion, and resin structure (e.g., pore size distribution) can all impact capture capacity, cycle lifetime, and moisture-swing behavior. (21−28) Resins contain counterions (e.g., chloride, carbonate, etc.) to maintain charge neutrality. (25,26,29) Recent studies have shown that increasing the NR4+ group density can result in a shorter half-time for CO2 capture. (27,28) In addition, phosphate counterions demonstrate a greater capacity for CO2 when compared with carbonate counterions. (30) Reversible hydrolysis and neutralization reactions at the cation–anion active site within an ion-exchange resin are necessary for moisture-swing CO2 capture. (31−33) Understanding how resin structure and anion type promote reversible hydolysis and neutralization within confined regions of sorbents remains an open question.
Here, we specifically aim to understand how the basicity of the counterion (e.g., anion) impacts moisture-swing direct air capture mechanisms. (32−34) Ion-exchange resins loaded with carbonate, phosphate, acetate, sulfide, and borate were synthesized and evaluated for the capture of CO2 and moisture-swing CO2 capture. This work reports two important discoveries that will help design better materials for moisture-swing CO2 capture. First, we experimentally demonstrate that anions with high basicity and moderate interactions with water (phosphate, carbonate, and borate) can enable reversible moisture-swing direct air capture. Second, we highlight the importance of water management on moisture-swing direct air capture. Macropores are important for facilitating water transport in sorbents and impacts the rate of the CO2 absorption. Understanding how the underlying anion–resin interactions impact moisture-swing direct air capture mechanisms can improve design strategies for next generation sorbent materials.
Basically (here a chemical pun) counterions, the active species for carbon capture, are investigated supported on quaternary amine resins. A figure from the text and the caption give some insight to the procedure:
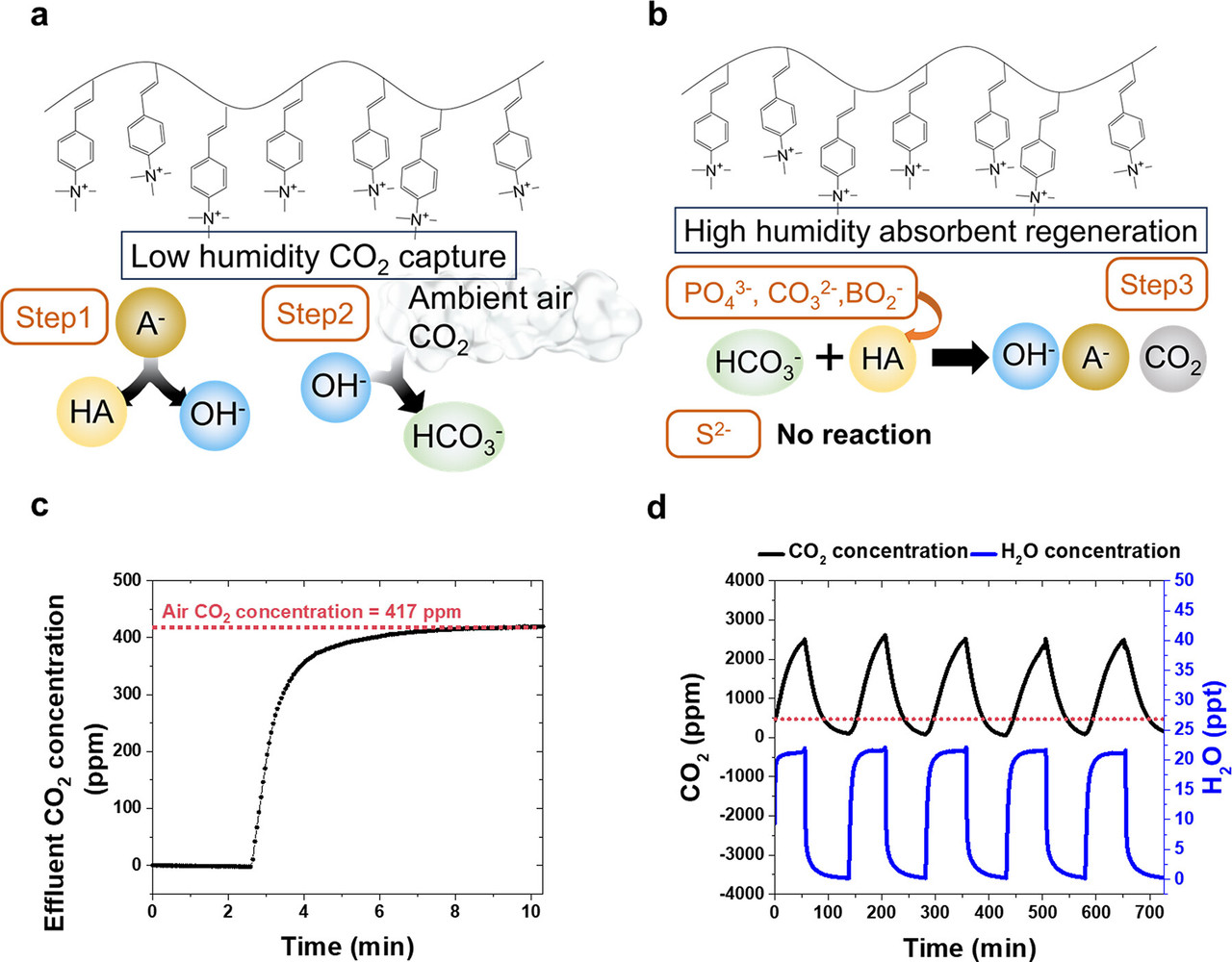
The caption:
Another bit of text from the paper is interesting and fun, since it gives an idea of how much resin is involved in capturing various amounts of carbon dioxide:
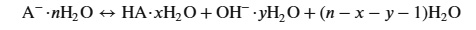
where A– represents anions (CO[SUB32–, PO43–, S2–, CH3COO–), and n, x, and y respectively represent the hydration numbers associated with the anion, the protonated anion, and the hydroxide.
A mmol of carbonate anion is approximately 0.0600 grams (CO[SUB32–), derived from 0.0440 grams of CO2. This process will never be industrial, but just like we pretend that so called "renewable energy" has a meaningful effect on the accumulation of the dangerous fossil fuel waste CO2 in the atmosphere - it doesn't - let's play pretend.
Detailed considerations of the amount of carbon dioxide dumped into the planetary atmosphere can be found at the Global Carbon Budget website. According to the data that can be accessed there, in 2024, the total amount of carbon dioxide added to the atmosphere from combustion of dangerous fossil fuels and forests, along with land use changes for agriculture, structures, and include industrial parks for wind and solar ripped out wilderness for what is disingenuously called "green" technology, and the mines to build that crap, was 37.615 billion tons, which translates to 37.6 X 1016 grams of the dangerous fossil fuel waste. The cycle time for the best resin, given in a figure not shown, indicates the half time for the capture is 50 minutes. This implies that in continuous operation, as a rough figure, about 5000 cycles per year could be carried out each year. This implies that we would need, at 0.0440 grams of CO2 per gram of resin per cycle, of 1.63 X 1014 grams of resin to address a single year of CO2 additions to the atmosphere as of 2024, assuming that the resin is capable of continuous use without degradation. This is equal to 163 million tons of resin.
Good luck with that.
(If the resin is made by the reduction of CO2 to methanol, followed by use of the MTO process to make aromatic functionalized alkenes to polymerize to resins, boy, could we sequester some carbon, couldn't we? This, if you missed it, is a sarcastic remark.)
It ain't gonna happen.
I do believe that under conditions in which a Brayton cycle with air as a working fluid, it may be industrially feasible using aqueous hydroxide solutions, preferably highly radioactive, for direct air capture, as a side product of Brayton nuclear powerplant operations, and I rant at my son about this idea from time to time, but perhaps this is no less pie-in-the-sky than the paper. Before we can remove carbon dioxide from the air, we have to stop adding it, and we are not even remotely close to doing so, since we value elaborate Rube Goldberg fantasies more highly than practical schemes.
I'm approaching the end of my life, and I regret what we have left for future generations.
If you are enjoying the President's Day holiday, even as we have a disgusting orange pedophile as President now, resulting in the complete fall and disintegration of our country, I trust it is going pleasantly anyway.
If you are not involved with the holiday, I trust you are having a nice day anyway.